Received: Wed 04, Dec 2024
Accepted: Wed 18, Dec 2024
Mini Abstract
This study examines the efficacy of ultrasound-guided, gene-based microwave ablation compared to surgery in managing low-risk papillary thyroid carcinoma. It highlights the critical role of genetic testing in tailoring treatment strategies, thereby improving patient safety and optimizing clinical outcomes.
Abstract
Objective: This study evaluates and compares the efficacy and prognosis of ultrasound and gene-based microwave ablation (MWA) and surgical treatment in patients with low-risk papillary thyroid carcinoma (PTC), emphasizing the influence of genetic mutations on low-risk patients’ selection.
Background: MWA, a minimally invasive technique, is increasingly recognized in the management of PTC. While traditional criteria for ablation focus on tumor size, number, and location, the impact of genetic mutations on treatment efficacy remains underexplored.
Methods: A total of 201 patients with low-risk PTC without metastasis were prospectively enrolled. All patients underwent ultrasound and next-generation sequencing to confirm low-risk status. Patients chose either ablation or surgery and were monitored until November 2024. Efficacy and complications were assessed using thyroid ultrasound and contrast-enhanced ultrasound.
Results: The median follow-up of this study is 12 months. There is no significant difference between the ablation group (3.0%) and the surgery group (1.0%), in disease free survival (DFS) (P= 0.360). However, the surgery group exhibited a significantly higher complication rate, particularly for temporary hypoparathyroidism (P < 0.001). Ablation offers notable advantages, including shorter treatment duration, faster recovery, less intraoperative blood loss, and reduced costs (P < 0.001), while maintaining favorable safety and comparable efficiency.
Conclusion: For patients with low-risk genetic mutations, ablation provides comparable efficacy and DFS to surgery, with with significant benefits in safety, recovery, and overall cost. Guided by ultrasound and next-generation sequencing, precise patient selection enhances the potential of ablation as a promising, minimally invasive alternative to surgery in the management of low-risk PTC.
Keywords
Papillary thyroid carcinoma, next-generation sequencing, microwave ablation,·surgical treatment
TABLE 1. Patients characteristics.
|
|
Ablation (N=100) |
Surgery (N=101) |
P value |
|
Age (year),
mean (SD) |
41.89 (11.13) |
40.79 (10.89) |
0.481 |
|
Gender (%) |
|
|
0.154 |
|
Male |
25 (25.0) |
17 (16.8) |
|
|
Female |
75 (75.0) |
84 (83.2) |
|
|
Tumor
location (%) |
|
|
0.295 |
|
Left |
51 (51.0) |
41 (40.6) |
|
|
Isthmus |
5 (5.0) |
8 (7.9) |
|
|
Right |
44 (44.0) |
52 (51.5) |
|
|
Tumor
morphology (%) |
|
|
0.912 |
|
Sharp margins |
51 (51.0) |
52 (51.5) |
|
|
Fairly clear margins |
16 (16.0) |
18 (17.8) |
|
|
Blurred margins |
33 (33.0) |
31 (30.7) |
|
|
Tumor aspect
ratio (%) |
|
|
0.301 |
|
<1 |
52 (52.0) |
49 (48.5) |
|
|
=1 |
4 (4.0) |
1 (1.0) |
|
|
>1 |
44 (44.0) |
51 (50.5) |
|
|
Maximum tumor
diameter (mm), mean (SD) |
6.14 (2.10) |
6.25 (2.11) |
0.696 |
|
Tumor volume
(ml), mean (SD) |
0.08 (0.09) |
0.08 (0.07) |
0.462 |
|
UItrasound
classification (%) |
|
|
0.484 |
|
TI-RADS 1 |
0 (0) |
0 (0) |
|
|
TI-RADS 2 |
1 (1.0) |
0 (0) |
|
|
TI-RADS 3 |
1 (1.0) |
2 (2.0) |
|
|
TI-RADS 4 |
28 (28.0) |
30 (29.7) |
|
|
TI-RADS 5 |
70 (70.0) |
67 (66.3) |
|
|
TI-RADS 6 |
0 (0) |
2 (2.0) |
|
|
CDFI (%) |
|
|
0.468 |
|
Blood flow signal within the nodule |
43(43.0) |
51 (50.5) |
|
|
Peripheral ring-like blood flow signal |
7 (7.0) |
4 (4.0) |
|
|
Ring-like blood flow signal within and around the nodule |
14(14.0) |
17 (16.8) |
|
|
No ring-like blood flow signal within and around the nodule |
36(36.0) |
29 (28.7) |
|
|
FNAB (%) |
|
|
0.505 |
|
TBS-I |
0 (0) |
0 (0) |
|
|
TBS-II |
0 (0) |
0 (0) |
|
|
TBS-III |
5(5.0) |
2 (2.0) |
|
|
TBS-IV |
0 (0) |
0 (0) |
|
|
TBS-V |
4(4.0) |
4 (4.0) |
|
|
TBS-VI |
91(91.0) |
95 (94.0) |
|
|
Gene mutation
type (%) |
|
|
0.664 |
|
Single BRAF gene mutation |
58(58.0) |
54 (53.4) |
|
|
BRAF gene mutation combined with other gene mutations |
37(37.0) |
38 (37.6) |
|
|
Other gene mutations |
2(2.0) |
5 (5.0) |
|
|
No gene mutation detected |
3(3.0) |
4 (4.0) |
|
|
Follow-up
time (month), mean (SD) |
11.51 (5.45) |
11.50 (3.12) |
0.434 |
|
Disease progression
(%) |
|
|
0.308 |
|
Yes |
3 (3.0) |
1 (1.0) |
|
|
No |
97(97.0) |
100 (99.0) |
|
CDFI: Color Doppler Flow Imaging; FNAB: Fine Needle Aspiration Biopsy; SD: Standard Deviation; TBS: The Bethesda System for Reporting Thyroid Cytopathology; TI-RADS: American College of Radiology Thyroid Imaging, Reporting and Data System.
TABLE 2. Patients
baseline between different gene mutations.
SD: Standard Deviation; TBS: The Bethesda System for Reporting Thyroid Cytopathology; TI-RADS: American College of Radiology Thyroid Imaging, Reporting and Data System.
TABLE 3. Disease
progression between ablation and surgery groups.
|
|
Ablation (N=100) |
Surgery (N=101) |
P-value |
|
Disease progression
(%) |
|
|
0.360 |
|
Cervical lymph node metastasis |
0 (0.0) |
0 (0.0) |
|
|
Local tumor progression |
2 (2.00) |
0 (0.0) |
|
|
Tumor recurrence |
1 (1.0) |
1 (1.0) |
|
|
Distant metastasis |
0 (0.0) |
0 (0.0) |
|
|
Recurrence
location (%) |
|
|
0.368 |
|
PTC-RT |
1 (1.0) |
0 (0.0) |
|
|
PTC-LT |
0 (0.0) |
1 (1.0) |
|
Local tumor progression, the tumor continues to grow within the ablation
zone.
PCT: Papillary Thyroid Carcinoma; PTC-RT: PTC of the Right Lobe; PTC-LT, PTC of the Left Lobe.
TABLE 4. Complications
and hospitalizations in ablation and surgery groups.
|
|
Ablation (N=100) |
Surgery (N=101) |
P-value |
|
Complication (%) |
|
|
<0.001 |
|
Postoperative fever |
0 (0.0) |
1 (1.0) |
|
|
Postoperative cervical pain |
0 (0.0) |
5 (5.0) |
|
|
Postoperative nausea |
0 (0.0) |
2 (2.0) |
|
|
Temporary hypoparathyroidism |
0 (0.0) |
19 (18.8) |
|
|
Postoperative hypertension |
0 (0.0) |
0 (0.0) |
|
|
Hemorrhage
(ml), mean (SD) |
0.00 (0.00) |
8.72 (3.74) |
<0.001 |
|
hospitalization
time (d), mean (SD) |
1.00 (0.00) |
2.88 (0.41) |
<0.001 |
|
Treatment
time (h), mean (SD) |
0.29 (0.04) |
1.31 (0.58) |
<0.001 |
|
Cost (CNY),
mean (SD) |
17669.73 (175.14) |
35757.29 (2781.39) |
<0.001 |
SD: Standard Deviation.

Study flowchart of patient inclusion for the study on low-risk PTC treatments at Sun Yat-sen Memorial Hospital. A total of 400 patients who underwent ablation or surgical treatment from January 2022 to November 2024 were initially screened for the prospectively observational cohort study. Exclusions included patients with multiple lesions or tumor diameters greater than 1 cm (n=123), those without malignancy indications in fine-needle aspiration biopsy (FNAB) and genetic testing (n=2), and patients with genetic testing indicating intermediate-high risk PTC (n=11). Additionally, patients with cervical lymph node metastasis (n=18) were excluded. Ultimately, 245 patients with low-risk PTC were included, with 141 opting for surgery and 104 choosing ablation. After further exclusions for incomplete data, the final groups included 101 patients in the surgery group and 100 patients in the ablation group.
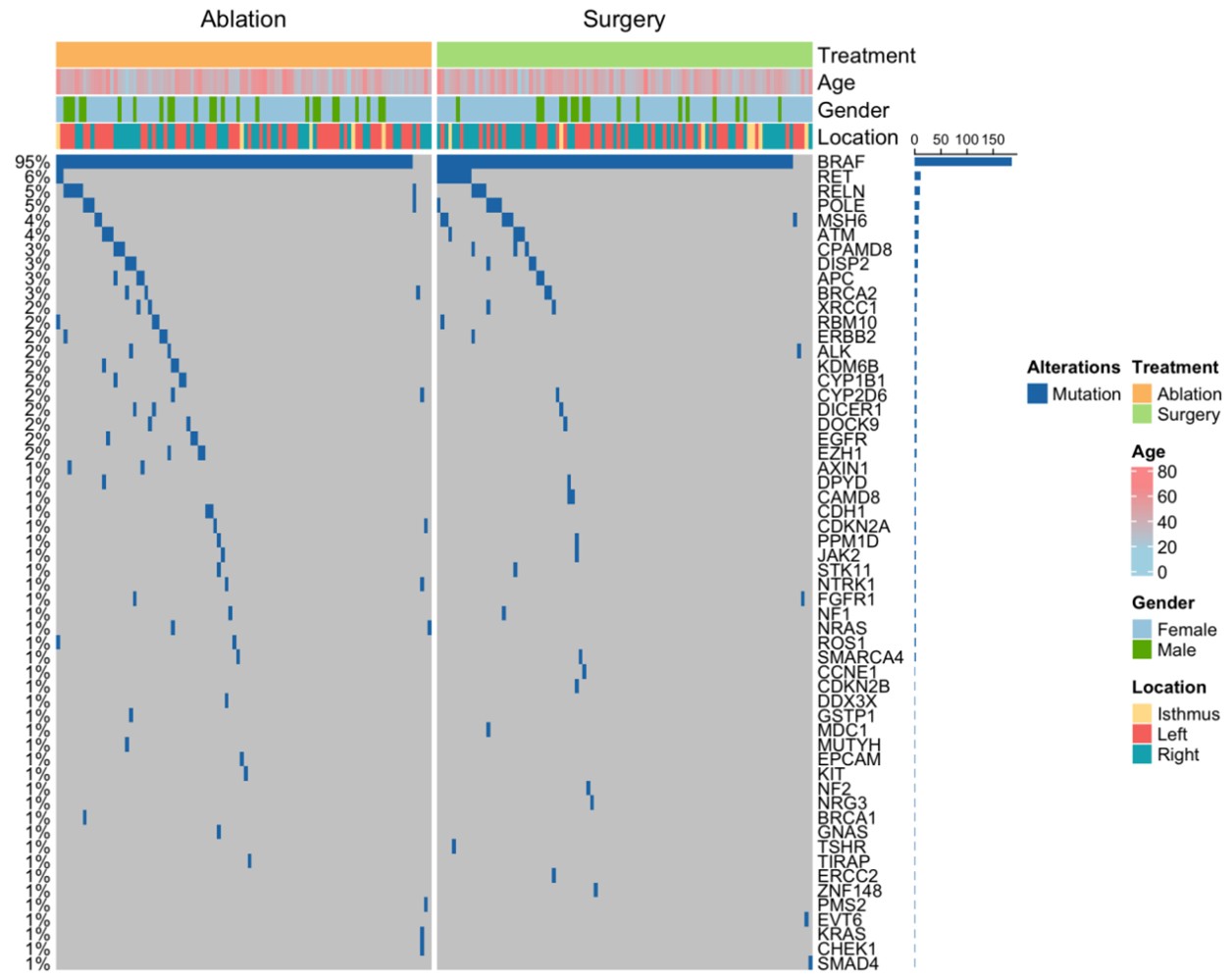
The figure shows the frequency of genetic mutations in patients undergoing ablation and surgery, with B-Raf proto-oncogene, serine/threonine kinase V600E (BRAF V600E) mutations being the most common.
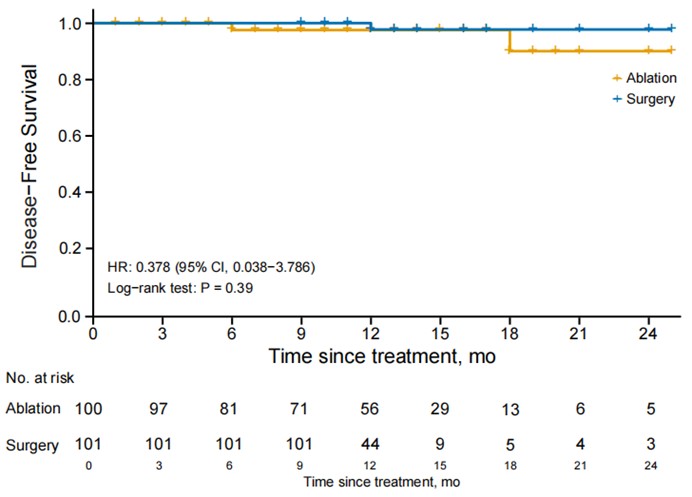
Kaplan-Meier curve illustrating disease free survival (DFS) for patients with low-risk PTC who underwent either ablation or surgery.

A) Pre-ablation thyroid ultrasound of patient A. B) Post-ablation thyroid contrast-enhanced ultrasound of patient A. C) Pre-ablation thyroid ultrasound of patient B. D) Post-ablation thyroid contrast-enhanced ultrasound of patient B. E) Pre-ablation thyroid ultrasound of patient C. F) Post-ablation thyroid contrast-enhanced ultrasound of patient C. G) Pre-ablation thyroid ultrasound of patient D. H) Post-ablation thyroid contrast-enhanced ultrasound of Patient D.
REFERENCES
1.
Rebecca L Siegel, Angela N Giaquinto, Ahmedin Jemal “Cancer statistics, 2024.” CA Cancer J Clin, vol. 74, no. 1, pp. 12-49, 2024. View at: Publisher Site | PubMed
2. Hyuna Sung, Jacques Ferlay, Rebecca
L Siegel, et al. “Global cancer statistics 2020: GLOBOCAN estimates of
incidence and mortality worldwide for 36 cancers in 185 countries.” CA
Cancer J Clin, vol. 71, no. 3, pp. 209-249, 2021. View at: Publisher Site | PubMed
3. Zubair W Baloch, Sylvia L Asa,
Justine A Barletta, et al. “Overview of the 2022 WHO Classification of Thyroid
Neoplasms.” Endocr Pathol, vol. 33, no. 1, pp. 27-63, 2022. View at: Publisher
Site
| PubMed
4. Gabriella
Pellegriti 1, Francesco Frasca, Concetto Regalbuto, et al. “Worldwide increasing incidence of thyroid cancer:
update on epidemiology and risk factors.” J Cancer Epidemiol, vol. 2013, pp. 965212, 2013. View at: Publisher Site | PubMed
5.
Junjie Huang, Chun Ho Ngai, Yunyang Deng, et al. “Incidence and mortality of thyroid
cancer in 50 countries: a joinpoint regression analysis of global trends.” Endocrine, vol. 80, no. 2, pp. 355-365, 2023. View at: Publisher Site | PubMed
6.
Iwao Sugitani “Active surveillance of low-risk papillary thyroid
microcarcinoma.” Best Pract Res
Clin Endocrinol Metab, vol. 37, no. 1, pp. 101630, 2023. View at: Publisher
Site
| PubMed
7.
Laura Boucai, Mark Zafereo, Maria E Cabanillas “Thyroid Cancer: A Review.” JAMA, vol. 331, no. 5, pp. 425-435, 2024. View at: Publisher Site | PubMed
8. Min Joo Kim, Jae Hoon Moon, Eun
Kyung Lee, et al. “Active Surveillance for Low-Risk Thyroid Cancers: A Review
of Current Practice Guidelines.” Endocrinol Metab, vol. 39, no. 1, pp.
47-60, 2024. View at: Publisher Site | PubMed
9. R Michael Tuttle, Duan Li, Fourat
Ridouani “Percutaneous ablation of low-risk papillary thyroid cancer.” Endocr
Relat Cancer, vol. 30, no. 3, pp. e220244, 2023. View at: Publisher Site | PubMed
10. Yusaku Yoshida, Kiyomi Horiuchi,
Takahiro Okamoto “Patients’ view on the management of papillary thyroid
microcarcinoma: active surveillance or surgery.” Thyroid, vol. 30, no.
5, pp. 681-687, 2020.
View at: Publisher Site | PubMed
11. Jung Heo, Hyun Jin Ryu, Hyunju Park,
et al. “Mortality rate and causes of death in papillary thyroid
microcarcinoma.” Endocrine, vol. 83, no. 3, pp. 671-680, 2024. View at: Publisher
Site
| PubMed
12. Mo-Han Guo, Jian-Ping Dou, Lin
Zheng, et al. “Ultrasound-guided microwave ablation versus surgery for solitary
T1bN0M0 PTC: a prospective multicenter study.” Eur Radiol, vol. 34, no.
1, pp. 569-578, 2024. View at: Publisher Site | PubMed
13. Ying Wei, Wen-Quan Niu, Zhen-Long
Zhao, et al. “Microwave Ablation versus Surgical Resection for Solitary T1N0M0
PTC.” Radiology, vol. 304, no. 3, pp. 704-713, 2022. View at: Publisher Site | PubMed
14. Chinese Medical Doctor Association
Thyroid Tumor Ablation Therapy Expert Group, Thyroid Cancer Committee of the
China Anti-Cancer Association, Ultrasound Interventional Professional Committee
of the Chinese Medical Doctor Association Interventional Physician Branch, et
al. (2018). Expert Consensus on Thermal Ablation Treatment for Benign Thyroid
Nodules, Microcarcinoma, and Cervical Metastatic Lymph Nodes (2018 Edition).
Chinese Journal of Oncology, vol. 27, no. 10, pp. 768-773, 2018.
15. Mengdi Jin, Zhijun Li, Yaoyao Sun,
et al. “Association analysis between the interaction of RAS family genes
mutations and PTC in the Han Chinese population.” Int J Med Sci, vol.
18, no. 2, pp. 441-447, 2021. View at: Publisher Site | PubMed
16. Jialong Yu, Yihan Zhang, Jian Zheng,
et al. “Ultrasound images-based deep learning radiomics nomogram for
preoperative prediction of RET rearrangement in PTC.” Front Endocrinol
(Lausanne), vol. 13, pp. 1062571, 2022. View at: Publisher Site | PubMed
17. Ziheng Ye, Xiaotian Xia, Peipei Xu,
et al. “The prognostic implication of the BRAF V600E mutation in papillary
thyroid cancer in a Chinese population.” Int J Endocrinol, vol. 2022,
pp. 6562149, 2022.
View at: Publisher Site | PubMed
18. Thitima Khonrak, Sasithorn
Watcharadetwittaya, Yaovalux Chamgramol, et al. “RET rearrangements are
relevant to histopathologic subtypes and clinicopathological features in Thai
PTC patients.” Pathol Oncol Res, vol. 29, pp. 1611138, 2023. View at: Publisher
Site
| PubMed
19. Athanasios Bikas, Sara Ahmadi,
Theodora Pappa, et al. “Additional Oncogenic Alterations in RAS-Driven
Differentiated Thyroid Cancers Associate with Worse Clinicopathologic
Outcomes.” Clin Cancer Res, vol. 29, no. 14, pp. 2678-2685, 2023. View at: Publisher
Site
| PubMed
20. Chinese Society of Endocrinology, et
al. “Guidelines for the Diagnosis and Treatment of Thyroid Nodules and
Differentiated Thyroid Cancer (Second Edition).” Chinese Journal of
Endocrinology and Metabolism, vol. 39, no. 3, pp. 181-226, 2023.
21. Bryan R Haugen, Erik K Alexander,
Keith C Bible, et al. “2015 American Thyroid Association Management Guidelines
for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The
American Thyroid Association Guidelines Task Force on Thyroid Nodules and
Differentiated Thyroid Cancer.” Thyroid, vol. 26, no. 1, pp. 1-133,
2016. View at: Publisher Site | PubMed
22. Jialong Liang, Wanshi Cai, Dongdong
Feng, et al. “Genetic landscape of papillary thyroid carcinoma in the Chinese
population.” J Pathol, vol. 244, no. 2, pp. 215-226, 2018. View at: Publisher Site | PubMed
23. Wei Guo, Junwei Huang, Taiping Shi,
et al. “Genotypes of PTC With High Lateral Neck Metastasis in Chinese
Population.” Front Oncol, vol. 12, pp. 816897, 2022. View at: Publisher
Site
| PubMed
24. Giovanni Mauri, Laszlo Hegedüs,
Steven Bandula, et al. “European Thyroid Association and Cardiovascular and
Interventional Radiological Society of Europe 2021 Clinical Practice Guideline
for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions.” Eur
Thyroid J, vol. 10, no. 3, pp. 185-197, 2021. View at: Publisher Site | PubMed
25. Lin Yan, Xinyang Li, Yingying Li, et
al. “Comparison of ultrasound-guided radiofrequency ablation versus thyroid
lobectomy for T1bN0M0 PTC.” Eur Radiol, vol. 33, no. 1, pp. 730-740,
2023. View at: Publisher Site | PubMed
26. Zhen-Long Zhao, Shu-Rong Wang, Gang
Dong, et al. “Microwave Ablation versus Surgical Resection for US-detected
Multifocal T1N0M0 Papillary Thyroid Carcinoma: A 10-Center Study.” Radiology,
vol. 311, no. 1, pp. e230459, 2024. View at: Publisher Site | PubMed
27. Lin Yan, WenHui Li, YaLin Zhu, et
al. “Long-term comparison of image-guided thermal ablation vs. lobectomy for
solitary papillary thyroid microcarcinoma: a multi-center retrospective cohort
study.” Int J Surg, vol. 110, no. 8, pp. 4867-4875, 2024. View at: Publisher
Site
| PubMed
28. Mingbo Zhang, Ralph P Tufano,
Jonathon O Russell, et al. “Ultrasound-Guided Radiofrequency Ablation Versus
Surgery for Low-Risk Papillary Thyroid Microcarcinoma: Results of Over 5 Years'
Follow-Up.” Thyroid, vol. 30, no. 3, pp. 408-417, 2020. View at: Publisher Site | PubMed
29. Lin Yan, Zhen Yang, Yingying Li, et
al. “Five-year Outcome Between Radiofrequency Ablation vs Surgery for
Unilateral Multifocal Papillary Thyroid Microcarcinoma.” J Clin Endocrinol
Metab, vol. 108, no. 12, pp. 3230-3238, 2023. View at: Publisher
Site
| PubMed
30. Lin Yan, Ying Liu, WenHui Li, et al.
“Long-term Outcomes of Ultrasound-guided Thermal Ablation for the Treatment of
Solitary Low-risk Papillary Thyroid Microcarcinoma: A Multicenter Retrospective
Study.” Ann Surg, vol. 277, no. 5, pp. 846-853, 2023. View at: Publisher
Site
| PubMed
31. Lin Zheng, Jian-Ping Dou, Zhi-Yu
Han, et al. “Microwave Ablation for Papillary Thyroid Microcarcinoma with and
without US-detected Capsule Invasion: A Multicenter Prospective Cohort Study.” Radiology,
vol. 307, no. 3, pp. e220661, 2023. View at: Publisher Site | PubMed
32. Julia E Noel, Sean M Wrenn “Outcomes
of Thermal Ablation for Papillary Thyroid Carcinoma.” JAMA Otolaryngol Head
Neck Surg, 2024. View
at: Publisher Site | PubMed
33. Lin Yan, Yingying Li, Xin Yang Li,
et al. “Clinical outcomes of ultrasound-guided radiofrequency ablation for
solitary T1N0M0 papillary thyroid carcinoma: A retrospective study with more
than 5 years of follow-up.” Cancer, vol. 129, no. 16, pp. 2469-2478,
2023. View at: Publisher Site | PubMed
34. Maria Sharmila Alina de Sousa,
Isabela Nogueira Nunes, Yasmin Paz Christiano, et al. “Genetic alterations
landscape in paediatric thyroid tumours and/or differentiated thyroid cancer:
Systematic review.” Rev Endocr Metab Disord, vol. 25, no. 1, pp. 35-51,
2024. View at: Publisher Site | PubMed
35. Rengyun Liu, Guangwu Zhu, Jie Tan, et al. “Genetic trio of BRAF and TERT alterations and rs2853669TT in papillary thyroid cancer aggressiveness.” J Natl Cancer Inst, vol. 116, no. 5, pp. 694-701, 2024. View at: Publisher Site | PubMed
